Abstract
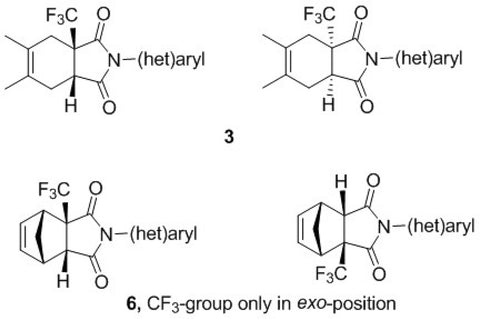
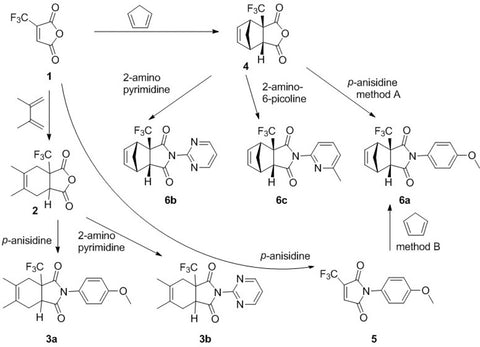
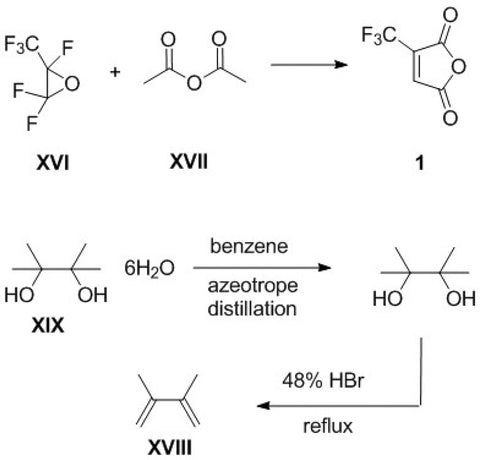
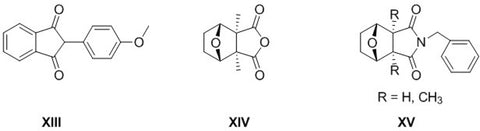
Novel trifluoromethylated mono- and bicyclic succinimides derived from trifluoromethylmaleic anhydride were synthesized using cyclopentadiene or 2,3-dimethylbutadiene and (het)arylamines. The biological activity of these compounds was evaluated using prediction methods and experimental studies. This series of new trifluoromethyl succinimides (3a,b and 6a-c) were tested by the National Cancer Institute (NCI, Bethesda, USA) by Program NCI-60 DTP Human Tumor Cell Line Screen at a single high dose (10-5 M). Imides revealed activity on Leukemia cell lines (RPMI-8226 - myeloma cell line), Non-Small Cell Lung Cancer cell lines (A549/ATCC - lung carcinoma epithelial cells) and Renal cancer cell lines (A498 and SN12C).
Keywords: anticancer activity; cycloaddition; cyclocondensation; fluorinated heterocycles; synthesis; trifluoromethyl succinimides; trifluoromethylmaleic anhydride.
Figures